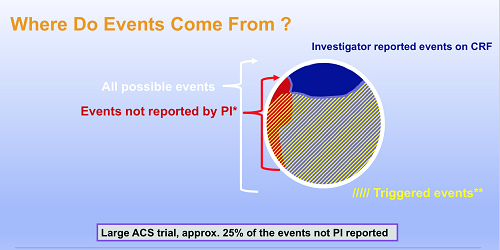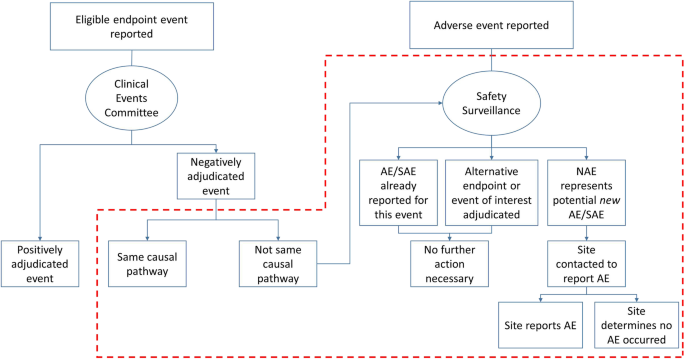
Methods for safety and endpoint ascertainment: identification of adverse events through scrutiny of negatively adjudicated events | Trials | Full Text
Inadequate planning and reporting of adjudication committees in clinical trials: recommendation proposal
Inadequate planning and reporting of adjudication committees in clinical trials: Recommendation proposal

Clinical events classification (CEC) in clinical trials: Report on the current landscape and future directions — proceedings from the CEC Summit 2018 - ScienceDirect

Abstract 16365: The Nature and Number of Unreported Events Identified by the Clinical Event Adjudication Process in the AEGIS-1 Trial | Circulation
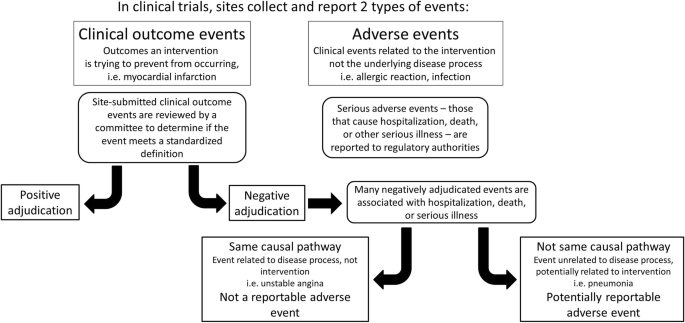
Methods for safety and endpoint ascertainment: identification of adverse events through scrutiny of negatively adjudicated events | Trials | Full Text